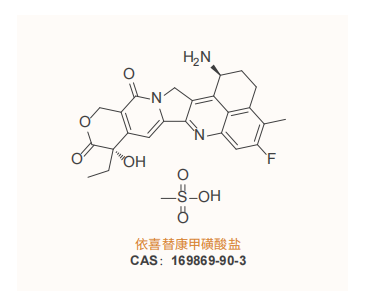
ChemExpress has successfully obtained the US FDA DMF filing for Exatecan mesylate
ChemExpress has successfully obtained the US FDA DMF filing for Exatecan mesylate, filing number: MF036708.
ChemExpress has completed the process development, confirmed batch production, analytical method development, stability study, and analytical method validation of Exatecan mesylate and its key intermediates. It can achieve stable production above kilogram level and can provide related registration declaration services.
In terms of structure confirmation, the ChemExpress R&D team has confirmed the absolute conformation of the structural backbone and chiral center of the molecule by single-crystal diffraction;
For the aspect of impurity control, a complete impurity control strategy and strict quality standards have been established to ensure the high purity and high quality of the product, based on the QbD concept;
In terms of intellectual property, we have independently developed a new process route, which not only broke through the relevant patent barriers of foreign companies but also took the initiative to carry out patent layout, including domestic CN111470998B (authorized), CN111875517A (sub-case), international PCT application WO2022000868A1, etc.
Deep cooperation with many companies domestic and abroad, as the payload infrastructure for extended research (route design, process development, quality standard formulation, clinical batch production, etc.), some customer projects have entered the IND application or clinical trial stage.
ChemExpress, one of the earliest companies in China to carry out ADC antibody conjugative drugs, has accumulated strong professional ability and rich research and development experience after years of scientific research and production practice. The company mainly provides CDMO services such as process development, CMC management, and product manufacturing of small molecule chemical materials related to ADC, including the development of toxins ( Drug ), connectors ( Linker ), and effective load ( Drug-Linker ), process optimization, process verification, registration declaration, and GMP industrialization.
We established a high-activity laboratory in Ma'anshan. Currently equipped with a 400m² super-kilogram GMP production line, which is equipped with four sets of HP-API isolators conforming to international OEL management specifications, suitable for the production of OEB5 (OEL <0.1μg/m³) compounds, meeting the needs of high-activity compounds including ADC small molecule toxins and payloads, including ADC small molecule toxins. The company will also actively invest in new production lines to meet the increasing demand for R&D and production consignment from different customers.