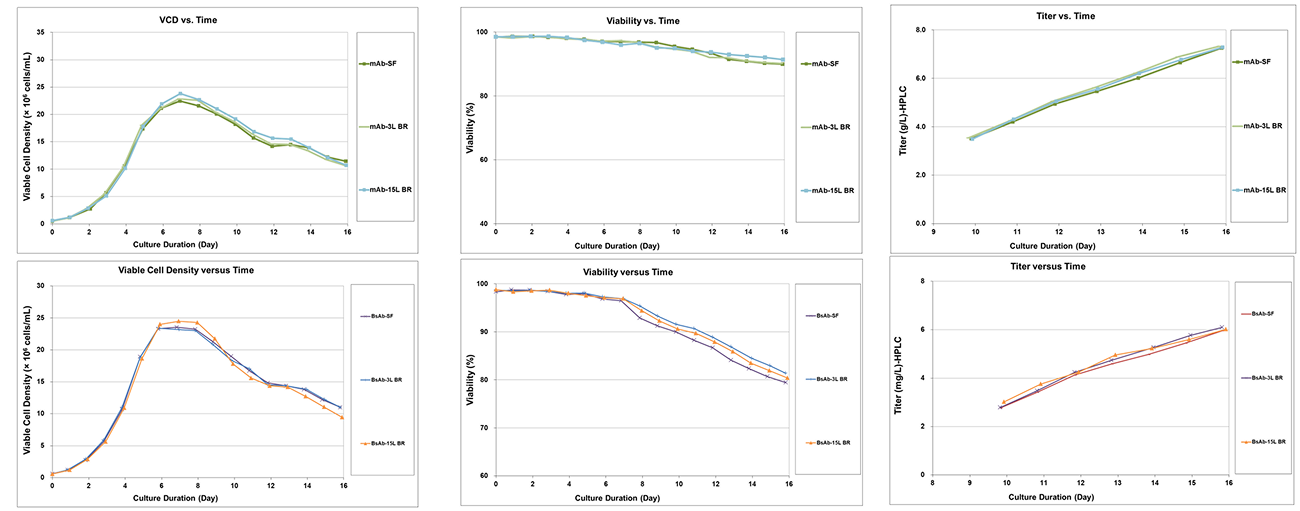
This project focused on process development for both mAb and BsAb cell cultures. Key findings are as follows:
· mAb cultured in CHO K1 (Horizon) cells achieved a peak viable cell density of approximately 2.2×10⁷ cells/mL, with a harvest viability of 91.1% and a final titer exceeding 7 g/L.
· BsAb cultured in CHO K1 (CHOZN) cells reached a peak viable cell density of approximately 2.5×10⁷ cells/mL, with a harvest viability of 80.3% and a final titer exceeding 6 g/L.
During upstream scale-up, from shake flask to 3 L bioreactor and further to 15 L bioreactor, both cell types demonstrated consistent growth profiles, viability trends, and productivity, confirming process robustness and scalability.
This project focused on the process development for improving the purity of both mAbs and BsAbs. Key findings are as follows:
· mAb: Yield >70%, SEC-HPLC purity >99%, charge variant (CEX) main peak >60%, HCP <10 ppm, endotoxin <0.1 EU/mg.
· BsAb: Yield >60%, SEC-HPLC purity >99%, charge variant (CEX) main peak >60%, HCP <10 ppm, endotoxin <0.1 EU/mg.
Overall, both mAb and BsAb downstream processes achieved high yield and high purity with well-controlled impurities (HCP, endotoxin), fully meeting the regulatory requirements for dual filing in China and the U.S..