Antibody Analytical Development
& Quality Control
In the golden era of antibody drug development, analytical methods have become the hidden champions that determine the success or failure of a drug—are you facing these challenges?
· Is method development like "navigating a maze"?—The complexity of antibody drugs makes traditional analysis service inefficient, delaying IND submission progress.
· Is compliance a "ticking time bomb"?—With frequent updates to FDA/EMA regulations, failing to validate analytical methods could result in clinical trial delays.
· Is product release a "tightrope walk"?—With significant batch-to-batch variability in antibody drugs, existing testing methods struggle to capture critical quality attributes (CQAs).
ChemExpress offers a comprehensive antibody analysis service! Leveraging cutting-edge technologies and industry expertise, we provide full support for the characterization and quality control of your antibody drug products, accelerating the path to market.

One-Stop Biologics
CDMO Service
We offer comprehensive, full lifecycle solutions encompassing monoclonal, bispecific, and multispecific antibodies, recombinant proteins, and ADCs, building an integrated service platform that covers the entire process from R&D to commercialization.

Expert Scientific Team
Our team of over 200 scientists provides comprehensive support from R&D to commercialization, enabling efficient scale-up and seamless technology transfer,effectively reducing management and transition costs.

Flexible & Efficient
Capabilities
We are equipped with reactor scales of 200L, 500L, and 2,000L, as well as precision instruments such as high-resolution mass spectrometers. Our production capabilities support all stages of drug development (Non-GMP & cGMP).
Our Services
Leveraging our proven platform processes, we accelerate the seamless transition of antibody therapeutics from proof-of-concept to large-scale batch production.
Our Service Scope Includes
Structural Characterization
Based on cutting-edge equipment such as Thermo Q Exactive high-resolution mass spectrometry, Waters Xevo G3 Q-TOF high-resolution mass spectrometry, PA800 PLUS, and Maurice capillary electrophoresis systems etc., ChemExpress Antibody Structural Characterization Platform provides comprehensive structural characterization services that meet ICH Q6B and FDA/EMA submission requirements, including:
- Primary Structure Analysis: Intact/Reduced/Deglycosylated Molecular Weight (error <5 ppm), N/C-Terminal Sequence Verification, Peptide Map Coverage ≥99%
- Post-Translational Modifications: Deamidation, Oxidation, Glycosylation, Disulfide Bond Mismatch (MS/MS Fragment Ion Confirmation)
- Advanced Structure: DSC Thermal Stability (Tm Value Deviation ±0.5°C), CD Secondary Structure, FT-IR Fingerprint Spectrum
Microbial Safety Control
ChemExpress Laboratory is CNAS-certified, equipped with Lonza Elx808 endotoxin analyzers and isolator sterile filling systems, providing:
- Bacterial Endotoxin: Gel Method (ChP General 1143) and Dynamic Colorimetric method (LOD ≤ 0.01 EU/mL)
- Microbial Limit: Membrane Filtration Method (Detects Aerobic Bacteria, Molds, And Yeasts)
- Sterility Testing: Isolator Technology
- Container Integrity Testing: Vacuum Decay Method (MD-750, Sensitivity ≥ 1 µm leak)
Stability Study
ChemExpress stability studies comply with ICH Q1A-Q1E and are designed in accordance with QbD principles:
- Forced Degradation: Oxidation (H₂O₂ 0.1%-1%), High Temperature (40-60°C), pH (3-9), Light Exposure (1.2 M lux•hr)
- The Freeze-Thaw Cycle (-80°C ↔ 25°C, 5 cycles) Simulates Clinical Usage Scenarios
- Real-Time Stability / Long-Term Stability (2-8°C, 24 months)
Equipment





Case Study
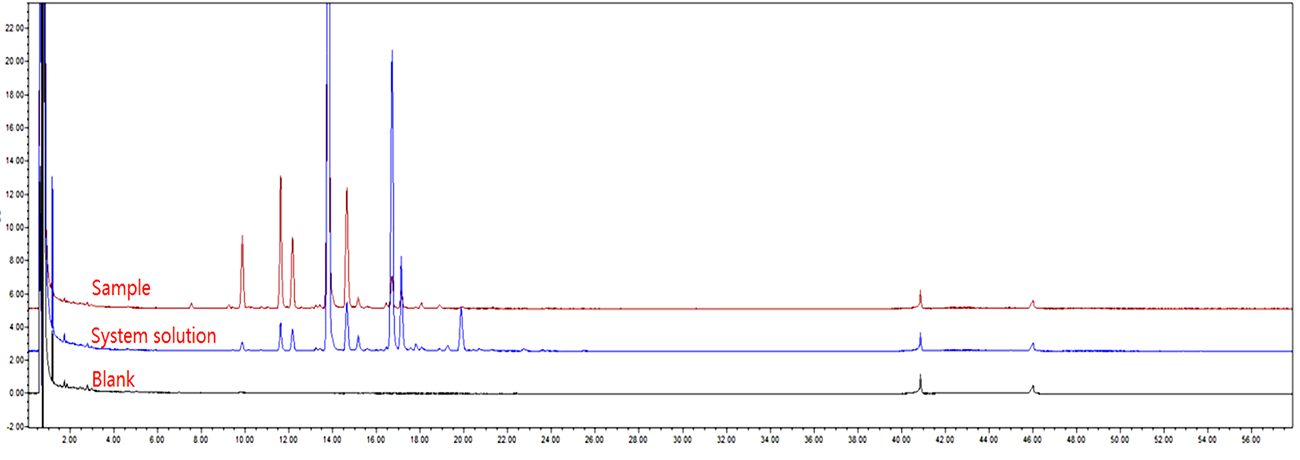
Glycan homogeneity is one of the critical quality attributes for antibodies. Glycan heterogeneity can affect the stability, activity, and immunogenicity of antibodies. Therefore, glycan profiling is an essential tool for ensuring the quality and consistency of antibody products.
At ChemExpress, we have developed an efficient antibody analysis service for glycan profiling of antibodies using HPLC and MS. This method systematically analyzes the composition and distribution of glycans within antibodies, ensuring precise monitoring of glycan heterogeneity.
By optimizing the analysis process, we successfully achieved:
· Efficient separation of different glycan variants
· Precise detection of glycan distribution, ensuring antibody consistency and stability
· Reliable data support for antibody quality assessment
FAQs
The gel-clot method is a semi-quantitative approach best suited for samples with high endotoxin concentrations.
The photometric method provides quantitative and precise results, making it ideal for low endotoxin level samples.
An isolator provides triple protection for operators, products, and the environment, making it ideal for high-risk samples such as live microbial preparations.
Its fully enclosed design minimizes the risk of cross-contamination and false positives.
When testing requires strict false-positive control, handling of high-risk materials, or complete process isolation, the isolator is the preferred solution.
The filter material should be compatible with the sample and solvent to ensure effective microbial retention and growth recovery.
Common membrane materials and their characteristics include:
· Mixed Cellulose Ester (MCE): High efficiency for aqueous samples; may adsorb inhibitory components.
· Nylon (N66): Suitable for most organic solvents and inhibitory samples.
· Polyethersulfone (PES): High flow rate and low protein binding; ideal for oily or protein-based samples.
· Polyvinylidene Difluoride (PVDF): Recommended for strongly inhibitory samples.
· Polytetrafluoroethylene (PTFE): Resistant to strong organic solvents such as DMSO.




